Clinical Trials and Studies
HHTU has a growing portfolio of studies at various stages of the research process – design, funding application, set-up and management. We work with a mixture of internal and external collaborators across a range of disease areas. We have demonstrated added value through the successful award of collaborative research grants, including NIHR-funded programmes of research.
We have and are collaborating with PIs all across the country to set up and run their studies, including many within the Hull area. We are also now starting to work internationally with our first partnership with an Italian team. Within all these studies we manage sites extremely widespread throughout the UK and soon to be Europe.
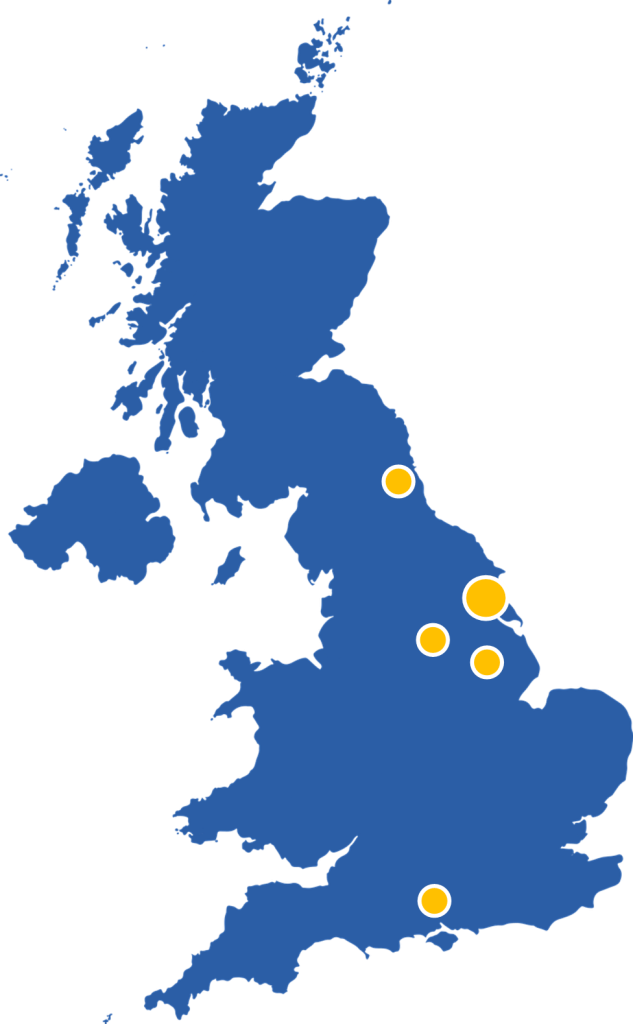
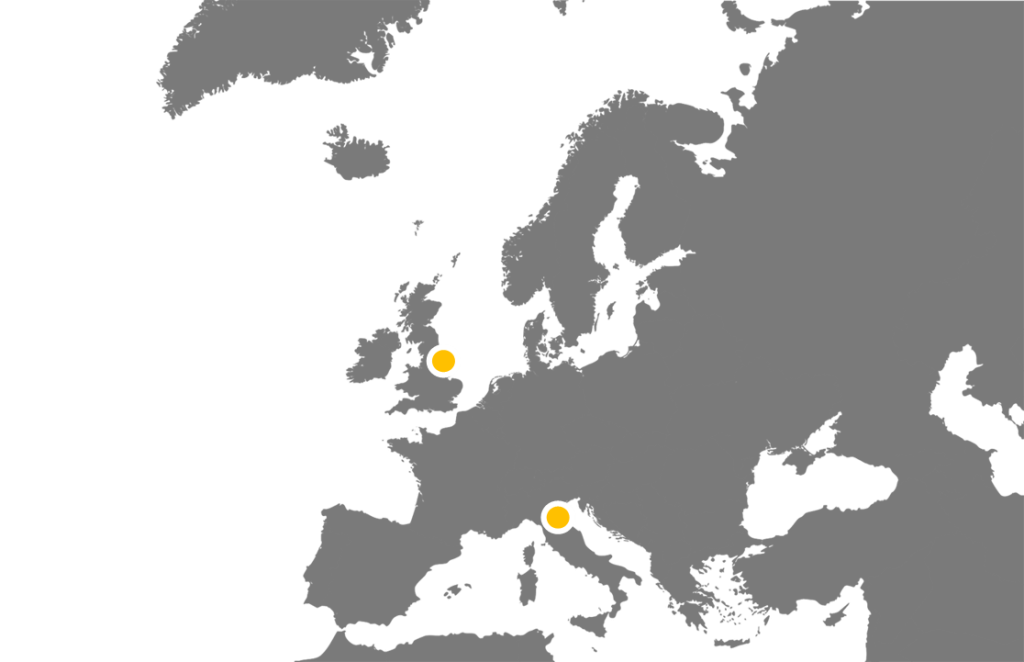
See the table below for the current portfolio of studies the HHTU is leading and collaborating on.
The table can be filtered using the search box or sorted by clicking the column headers.
| Study Name | Disease Area | Funder | Status |
|---|---|---|---|
| COPD Cardio Protect | Respiratory | Astra Zeneca | In set up |
| FANFIRST | Respiratory | NIHR RfPB | In set up |
| DOORstep | Cancer / Early Diagnosis | Yorkshire Cancer Research | In set up |
| CANFit | Cancer | Yorkshire Cancer Research | Recruiting |
| MOI-A | Rare Disease | UKRI | Recruiting |
| PRETZCEL | Cancer | ImaginAb | Recruiting |
| STARLIT | Endocrinology | Medical Research Council | STARLIT 1: Analysis/publication STARLIT 2: Recruiting STARLIT 3: In set up |
| ProACTIVE | Alcohol | NIHR HSDR | ProACTIVE WP1: Analysis/publication ProACTIVE WP2: Recruiting ProACTIVE WP3: In set up ProACTIVE WP4: In set up |
| SENTINEL QUAL | Respiratory | Astra Zeneca | Recruiting |
| PRE-DX | Cancer (breast) | Exact Sciences | In follow-up |
| MABEL | Respiratory | NIHR HTA | In follow-up |
| REDUCE | Mental Health / Primary Care | NIHR PGfAR | Analysis/publication |
| EXAC-QUAL | Respiratory | Astra Zeneca | Analysis/publication |
| FASTer | Mental Health | OPCC and Y&H CRN | Analysis/publication |
| CHAMPION | Respiratory | NIHR RfPB | Analysis/publication |
| NEWDAY ABC | Cancer (breast) | Yorkshire Cancer Research | Analysis/publication |
| LIPS | Cancer (blood) | Blood Cancer UK | Analysis/publication |
| BREEZE-IPF | Respiratory | NIHR RfPB | Analysis/publication |
| BREATHE | Respiratory | NIHR RfPB | Closed |

